Have you ever stopped to consider how legible your essential prescribing information is on your promotional materials, or even what is meant by legible in this context?
The legislation governing the advertising of medicines in Ireland (The Medicinal Products (Control of Advertising) Regulations 2007; S.I. No. 541 of 2007) requires full advertisements aimed at persons qualified to prescribe or supply to contain essential information compatible with the summary of product characteristics. Some of this essential information is presented within the advertisement itself, whereas other information (due to volume) is assimilated and presented within abbreviated prescribing information (API). The API typically contains:
i. A clear statement of entries from the SmPC relating to dosage and method of use relevant to the indication(s) advertised;
ii. Details on the method of administration if not obvious;
iii. Clear information on adverse reactions, precautions, and relevant contraindications.
This information “shall be printed in a clear and legible manner”(1).
An article from 2015 published by the Nielsen Norman Group, a Research-Based User experience consultancy, summarised that “Users won’t read web content unless the text is clear, the words are simple, and the information is easy to understand.”(2). This holds true not only for online materials but also printed pieces as well. NNG goes on to define “legibility” as:
“Legibility is the lowest-level consideration in content usability: it’s whether people are able to see, distinguish, and recognize the characters and words in your text. Legibility is thus mainly determined by visual design, specifically typography.”2
So what has this got to do with prescribing information on advertisements?
While you may be confident in meeting the provision of information requirements, how satisfied are you that your API is legible? Open any medical press publication and look at the medicines advertised therein. You will notice there is a vast difference in the approaches companies take in addressing this. Very often when creating an advertisement, the focus is on ensuring the advertisement addresses brand strategy, and that all the relevant information is in the advertisement itself; little attention may be paid to the API.
Considerations
The aim of the API is to provide the relevant information in a succinct manner, so that it is easily digestible by healthcare professionals. The API is critical as it contains the relevant information for healthcare professionals to ensure patient safety. If your API runs the full width of the page – will the healthcare professional be able to follow the text easily and identify the relevant pieces of information they need? If it is broken down into columns, which are more digestible in format(3), does this enhance the reader’s experience and their ability to absorb the relevant information? The University of Washington have a great example which demonstrates the importance of this – see the example taken from their website below(3). They also explain the importance of readability versus legibility alone.
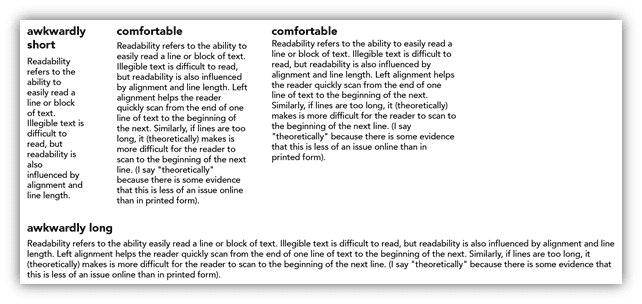
Image Source: https://canvas.uw.edu/courses/966147/pages/analyze-legibility-and-readabilityn
Font size is also important, as is the media on which it is published. What may look fine on screen and may be easily read on an A4 piece, may not be as easy to make out on something that is A5. Some companies designate a minimum font size for the API, which can be very useful for providing guidance to originators and reviewers alike. It also highlights the importance of double-checking the final printed material. Another consideration is whether the ad is in print or digital format, many publications have both online and print versions of each issue.
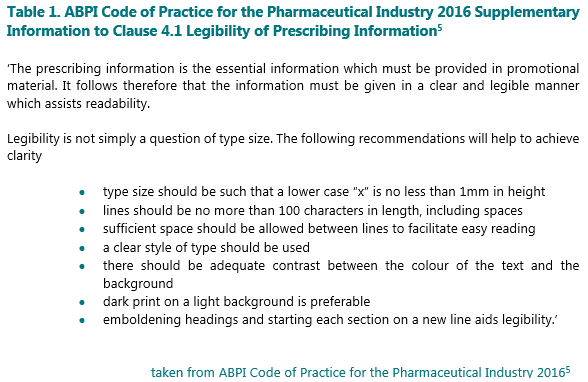
While the latest 2021 version of the ABPI Code in the UK states only that prescribing information be provided in a clear and legible manner (s.i. to Clause 12.1)(4), older versions of the Code (prior to 2019)(5) had specific recommendations on font size and format (see Table 1 below). While the changes in the recommendations no longer mean you need to have your ruler on your desk to ensure your x is no less than 1mm in height, the general principles are worth considering. The IPHA Code in Ireland stipulates only that information “must be given clearly and legibly”(6) so companies may wish to develop their own internal standards for use across all brands and therapy areas.
So what considerations are there when creating or reviewing the next promotional material requiring an API which comes across your desk?
Final format of the advertisement
- Will it be printed or digital? Will more than one print size be needed? If so, separate jobs will be required per unique presentation, and the API will need to be assessed as an integral part of the individual advertisements.
Headings
- Embolden and/or underline headings or sections. This helps the reader to easily navigate content.
Font
- The font type – the choice of specific font has a significant impact on legibility.
- The colour of the text – make sure there is sufficient contrast with the background colour, and that readability isn’t affected by artwork in the background.
- Size of text – large enough to be read comfortably. Consider an internal minimum font size.
Line length or column size
- How long is the string of text? If you have got distracted or nodded off half-way through then it is worth considering dividing your API into narrow columns. This has the added advantage of looking more aesthetically pleasing on the advertisement, as well as being easier to read.
Version control
- API should be presented within its own unique identifier, and the date on which the particulars were generated or last updated.
Whatever format you choose ensure that everyone is briefed on the minimum standards, including any third-party agencies developing content on your behalf. It is worth remembering that the purpose of the API is to provide essential information to Healthcare Professionals to support the rational use of the advertised medicinal product.
If you are interested in discussing topics such as this, and sharing your experience with your peers in other pharma companies, why not become a member of the Pharma Integrity Compliance Network? The Pharma Integrity Compliance Network is a Network of pharmaceutical professionals with an interest in compliance who meet several times a year to discuss hot topics in the area. For more information on joining contact info@pharmaintegrity.com.
Laragh de Bhulbh
References
1. S.I. No. 541/2007 – Medicinal Products (Control of Advertising) Regulations 2007
2. https://www.nngroup.com/articles/legibility-readability-comprehension/ (Last accessed 26th June 2021)
3. https://canvas.uw.edu/courses/966147/pages/analyze-legibility-and-readability (Last accessed 26th June 2021)
4. ABPI Code of Practice for the Pharmaceutical Industry, 2021
5. ABPI Code of Practice for the Pharmaceutical Industry, 2016
6. IPHA Code of Practice for the Pharmaceutical Industry, v8.5


