The HPRA issued a notification in Nov 2020 of their intent to establish a specific compliance programme assessing advisory boards taking place in ROI, and those held outside ROI but which involve Irish experts (HCPs, patients, and others). Updated criteria for advisory boards in scope of this programme were circulated in Feb 2021, and are described below:
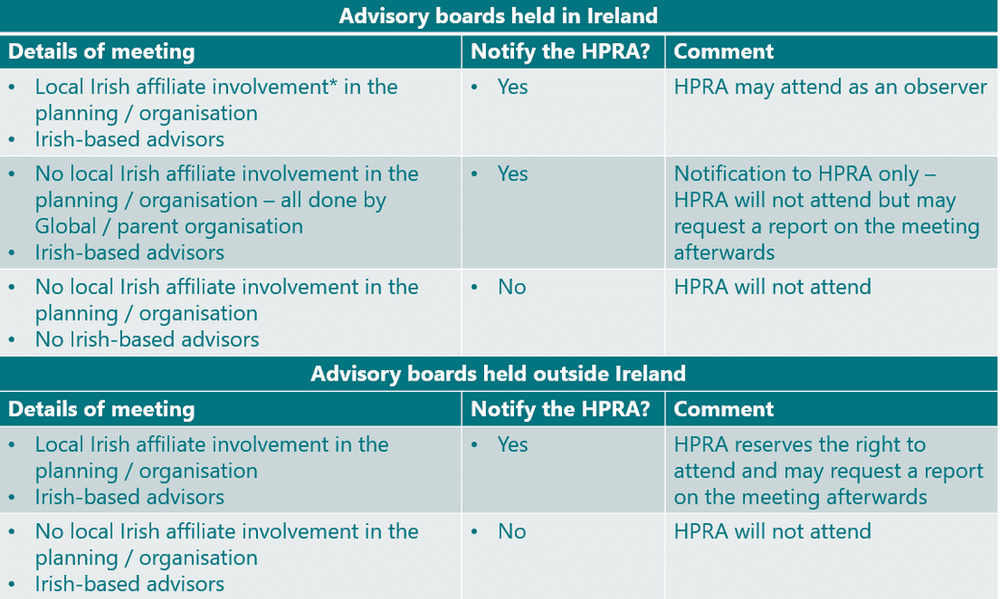
*Note: Local Irish affiliate involvement includes any of the following:
- Planning the advisory board
- Selecting the advisors
- Issuing invitations to advisors on its own letterhead / email, etc.
- Involvement in any logistical arrangements, e.g. making or assisting with travel or accommodation arrangements, venue hire, hospitality, provision of a medical writer, etc.
- Hosting the advisory board
- Running the advisory board
- Attending the advisory board
- Paying the advisors
The HPRA should be notified at least six weeks in advance of the proposed date for an advisory board meeting. Advisory boards that take virtually are within scope and subject to prior notification.
The HPRA have detailed the information that should be provided on their website, and have provided a notification form which may be accessed here.
Pharma Integrity has provided a free of charge notification template for MAHs to use when submitting information to the HPRA. All of the the HPRA’s stipulated requirements are reflected, and the format has been designed to be user friendly for providing information, and receiving (potential) comments or responses from the HPRA. You can download the Pharma Integrity template here:


