Do you have a Core Claims Document (CCD) for your product or brand? If so, do you use it? nThe use of CCDs (sometimes also referred to as ‘brand books’) is a topic that has come up a few times recently in dialogue with clients. At the heart of it, a CCD can be an extremely useful tool for a brand team with the potential to reduce the amount of work in creating and reviewing promotional materials.
In brief, a CCD is a live document created and maintained by the brand team with all the relevant core claims and obligations to that product or brand. As a living document it should be updated throughout the lifecycle of the brand/product as claims and safety data evolve. The idea is that the CCD is created, referenced and submitted for review, and thus provides a central reference for the core ‘approved claims’ and data on which they are based for all promotional material. Companies can document any approval commitments that they are subject to, e.g. as part of the product’s risk minimisation plan, and any inter-company agreements or undertakings that the company has committed to should be logged for prosperity. This ensures that when individuals move away from brands, there isn’t a loss of corporate memory as to commitments that have been made. For companies who adopt a CCD, it provides a quick reference guide for all previously approved claims and other ‘need to know’ information.
How to create and maintain a CCD
For a CCD to provide value, it needs to describe the key claims built upon the safety and efficacy data in pivotal trials. These claims are used repeatedly to underpin campaigns. As the product moves through its lifecycle and more data is released, through post-marketing studies and post-marketing surveillance, the CCD should be updated accordingly. Early in the lifecycle and at times when new indications are launched, the CCD should be reviewed and approved for accuracy on a regular basis, for example, every 6 months. This is particularly relevant where new data cuts are released and published (for example, when a peer reviewed paper becomes available instead of a congress abstract). As the product becomes more established and there are less studies being published, the review period can be extended, for example an annual review. It is worth checking to see if global colleagues have already created one before initiating one locally. Ensure that any such document is fairly balanced, and that both safety and efficacy claims are captured.
When it can be of benefit
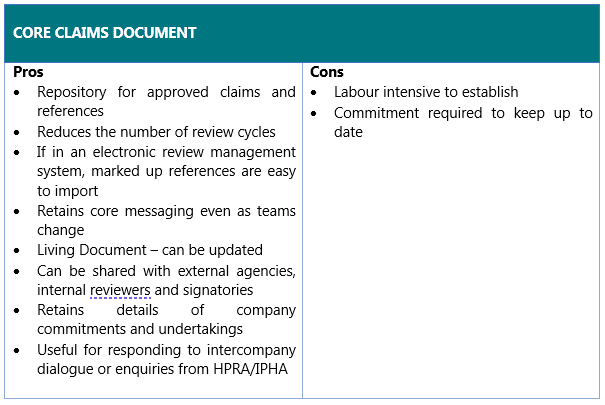
By having a CCD you can reduce the burden, number of review cycles and time spent creating material as your claims have already been pre-approved. The CCD can be shared with the brand team, external agencies, reviewers and signatories. This is extremely valuable as it retains the agreed upon messages as people move teams. By sharing it within a team it allows all members of the team to be aligned on the key messages for the brand. In an instance where there is intercompany dialogue or discussions ongoing with a regulatory authority, a CCD can be easily retrieved and used as a key single internal reference for formulating responses. During audits or inspections, it also shows that the required due diligence has been applied when formulating claims.
The drawbacks
The CCD is a living document and therefore the responsibility lies within the brand team to maintain the document and ensure that it accurately reflects the latest available data. There may be a significant investment of time initially to generate the core claims and to have them approved, however, the maintenance of the document should be less labour intensive. If the CCD is approved within an electronic review system such as Veeva PromoMats, then this both acts as a repository for the document and allows teams to easily access the references on which the claims are based, when needed. If the CCD is not regularly reviewed, then relying on this as the basis of a promotional claim could lead to out of date data being used as a reference which is misleading.
In summary a CCD could be a valuable tool for your brand team. For further information or guidance on how to create and maintain a CCD, or for help in drawing one together for your brand, contact us at Info@PharmaIntegrity.com