When creating or reviewing promotional print advertisements as part of a campaign the requirements for what information can and must be included are clear. What about digital banner advertisements, though? There are a number of considerations that are unique to digital banner advertisements, rather than print.
Digital banner advertisements come in a variety of shapes and formats. The imagery can be static or it can rotate through a number of different frames. As banner advertisements are generally only a small portion of the screen there are limitations to the amount of information you can provide. Despite this banner ads can be “full” or “reminder” advertisements dependent on content.
Audience and access
Who is the intended audience? Is this clear from the ad itself? When creating and approving an advertisement you need to consider where the banner ad is going to be hosted and who will be able to view it. You will need to confirm that the content of the advertisement is restricted to the intended audience and if for example it is targeted at prescribers of prescription only medicines then the general public should not be able to access it. Some companies clearly outline on the advertisement itself that it is for healthcare professionals only.
“Reminder” or “full” advertisement
A banner advertisement can be a reminder or a full advertisement, provided all legal and code obligations are fulfilled. It is worth keeping in mind that imagery within an advertisement can be considered a claim, and therefore must be considered appropriately when assessing and confirming whether it supports the rational use of the medicine, and the correct categorisation of the advertisement.
Obligatory information
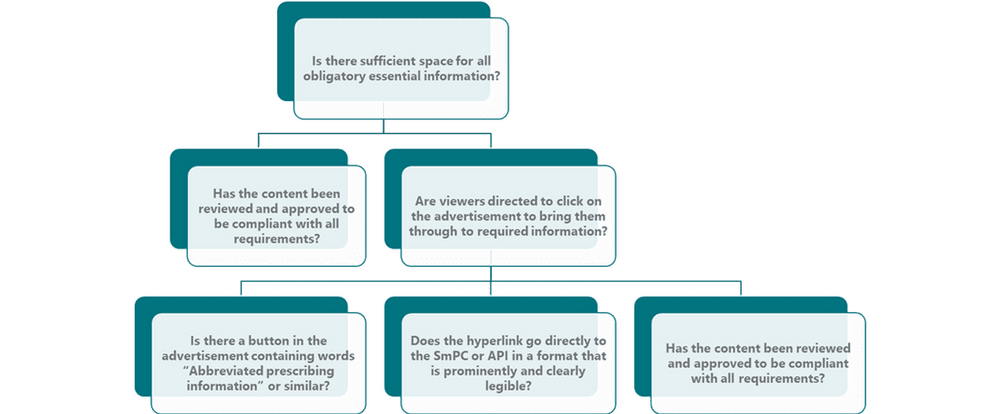
Under Irish advertising legislation and the IPHA Code, essential information must be included in all promotional materials and advertisements. With a banner advertisement space is at a premium, so considerations must be made as to whether it is a reminder advertisement, a full advertisement, and whether it is static or dynamic. No matter the approach selected, the HCP must be able to access the essential information. See figure below for further information. The first question is whether there is sufficient space to include the requisite information on the ad itself. In the instance where there isn’t space, the IPHA Code states that “…viewers must be either directed to click on the advertisement to bring them through to the required information, or a ‘button’ included on the advertisement contain the phrase “Abbreviated Prescribing Information” or similar.” It is also required that the button or relevant direction must be prominent and clearly legible on the advertisement. Any hyperlink must lead directly to the SmPC or abbreviated prescribing information (aPI) which must be in a format which is clearly legible. For more information on legibility and aPIs take a look at our past post on this topic here. The IPHA Code recommends that banner advertisements link directly to the SmPC on www.medicines.ie. The advantage of linking to directly to www.medicines.ie is clear: all prescribing information is up to date and all relevant information required is stored here, thereby reducing the risk of linking to an outdated aPI.
Hyperlink
Another consideration is whether hyperlinks are contained within the advertisement. This may be a link to the prescribing information or to a company website. In all cases the company creating the advertisement is responsible for the content which is linked.
Dynamic or static
Is the advertisement dynamic, i.e. does the content and the imagery change over a series of frames? This is something that must be carefully considered. If creating a dynamic banner advertisement you must ensure you consider the content not only of each frame but also whether all frames will function on all browsers. A case brought to the PMCPA for consideration in the UK AUTH/3148/1/19 & AUTH/3179/4/19 – Complainant v GSK; Online promotion of Seretide highlighted an issue with access to dynamic banner advertisements when the viewer is using old internet browsers. GSK had created a dynamic digital banner advertisement which had been certified as a transitioning four frame advertisement. Unknown to GSK, the third-party digital agency that developed the advertisement had arranged that the second frame be the “static” back up image should older browsers limit the advertisement from appearing dynamically. Frame 2 contained the product name twice, once as a heading, second as part of the logo at the bottom of the frame. The INN appeared as part of the logo but this was not the first appearance of the brand name. The PMCPA ruled that the company were in breach of Clause 4.3 (provision of essential information) and Clause 14.1 (Certification; 2016 ABPI Code). When creating and certifying banner advertisements this is a consideration that developers and approvers must take into account: “Will this advertisement appear as is on all browsers, or is there any potential variation?”.
Summary
Regardless of whether your final advertisement is a full ad or a reminder ad, or whether it is dynamic or static, the key issue is whether you have fulfilled your obligations under Irish advertising legislation and the IPHA Code regarding the provision of essential information. If you are interested in discussing similar topics with like minded colleagues contact Info@PharmaIntegrity.com to join the Pharma Integrity Compliance Network. If you are looking for information or guidance on this or similar topics contact us at Info@PharmaIntegrity.com.
Laragh de Bhulbh, Healthcare Compliance Consultant, Pharma Integrity


