Last month, the disclosable 2019 transfers of value (TOVs) made by 41 IPHA member companies and five non-member companies were made public on the transferofvalue.ie website. Pharma Integrity conducted an analysis of the data that was made public and available on 11th July 2020, and identified the following points of interest:

HCOs
A total of 818 HCOs were listed as recipients of TOVs. Of these, 618 had more than one TOV disclosed to them, but this is likely to be an imperfect reflection of payments made due to variances in data entry by companies. For example, TOVs to the Mayo branch of the Irish College of General Practitioners (ICGP) were recorded as “Mayo Faculty of the ICGP”, “Mayo ICGP”, “Icgp Mayo”, and “Mayo Faculty ICGP”. Individually these were for payments of €1,500 each but collectively the total for 2019 was €6,000. In looking at the central body of the ICGP, there were five different permutations of the association’s name: “Irish College General Practitioners (ICGP)”, “Irish College of GPs (ICGP)”, “ICGP”, “ICGP Board”, “Irish College of General Practitioners (ICGP)”. Collectively, TOVs totalling €53,174 were made, but there is a possibility that some companies may have erroneously disclosed payments to local chapters to the central association. When looking at TOVs to local chapters of the ICGP, there is a range in the value of single company payments made from €205 (paid to ICGP South West by Mundipharma) to €6000 (paid to ICGP Kildare also by Mundipharma).
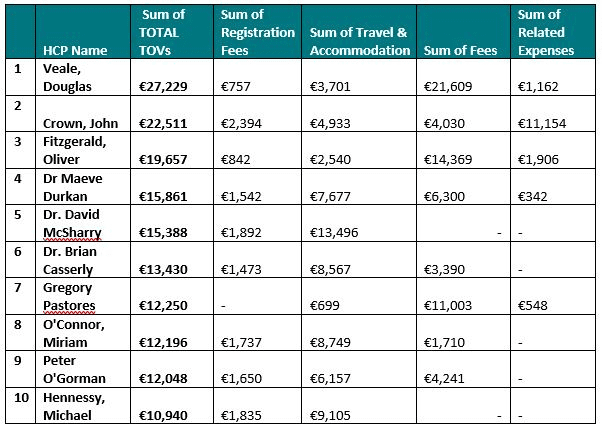
Notably, the 15 HCOs that received the greatest amount of TOVs by value from pharma companies in 2019 were located in Dublin. By value, the 10 HCOs that received the greatest total value of TOVs across all reportable categories were:
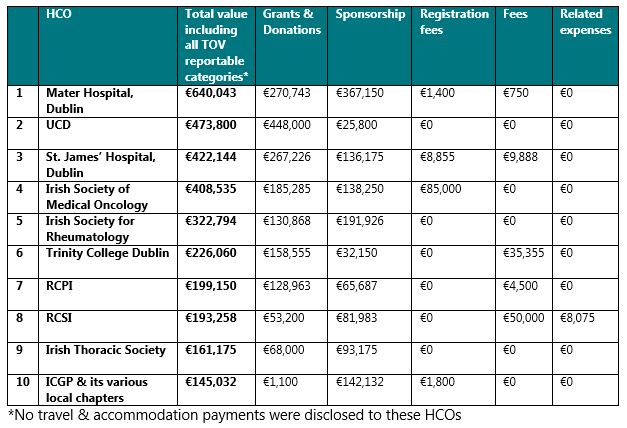
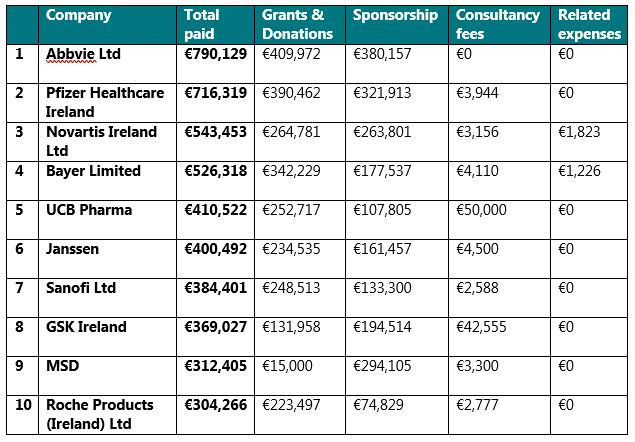
The companies that paid / provided the highest amount of disclosable TOVs to HCOs in 2019 were:
R&D Payments
The companies that disclosed the highest amount of R&D associated transfers of value were:
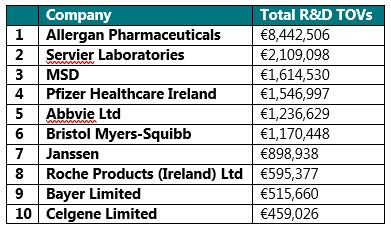
We currently have no additional detail with regards what these payments were associated with, however there have been discussions within EFPIA as to whether moving forwards, payments associated with R&D should be made more transparent. It is therefore highly likely over the next five years that changes will be made to what is disclosed, and how companies across Europe disclose these payments.
HCP Payments
It is very difficult to make an accurate analysis, or draw fair conclusions over the TOVs disclosed to individual HCPs. This is because each company’s disclosure on the transferofvalue.ie website is a little different. The IPHA Code disclosure template replicates the EFPIA standardised disclosure template. This calls for companies to provide the “Full name” of HCPs in the first column of data, followed by information on where they practice and a breakdown of payments according to category (contributions to costs of events and fees for service and consultancy). Some companies lead with a title, (e.g. Dr Jane Smith), some lead with first name (e.g. Jane Smith), others lead by surname (e.g. Smith, Jane or Smith, Dr Jane), and this is before we even begin to consider typos and spelling discrepancies (e.g. Jane vs Jayne, Smith vs Smyth). This issue doesn’t occur in the UK as the ABPI’s disclosure template separates each of these pieces of data into individual columns (Title / First name / Surname) and, using a third party service provider, the ABPI perform a “data matching” review of company submitted data, which typically occurs at the beginning of Q2 each year. The third party service provider assesses the data, groups all data for any given individual HCP together (based on a combination of name, place of work, email, local register ID, and third party database ID), and checks on individual discrepancies (including phoning individual places of work).
A total of €4,295,375 in transfers of value to named individual HCPs were disclosed by all companies collectively in 2019. This can be broken down to €890,406 for registration fees, €2,363,696 for travel and accommodation, €856,342 for consultancy fees, and €184,931 for related expenses. There was a total of €2,325,861 disclosed as aggregate payment (€400,208 total registration fees, €1,044,440 travel and accommodation, €743,583 consultancy fees, and €137,630 related expenses). Pharma Integrity assesses that in 2019, the percentage of payments disclosed to named individuals was 63%, slightly down from 64% in 2018. It is well known that IPHA are exploring the use of “legitimate interest” as the legal basis for processing and disclosing TOV data with the next iteration of the Code. By removing the need for consent (as has previously been done in Spain), we should have much greater transparency in future payments made to individual HCPs.
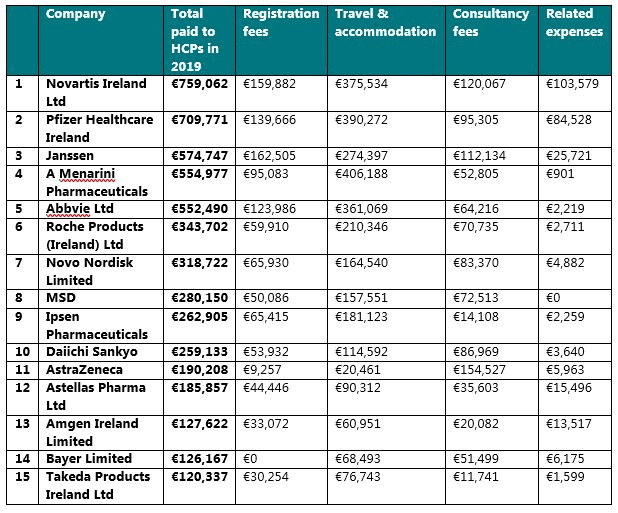
15 companies disclosed that they had provided TOVs of more than €100,000 in total to HCPs in 2019 (named individual and aggregate). These were:
Of those who were named in the individual disclosure report (but not taking into account that there may be duplicated entries based on variances in data entry and typos, etc.), the ten individual HCPs who consented to disclose the greatest value of TOVs in total across all the disclosure categories are:
Professional Conference Organisers
2019 was the first year in which data on payments made to Professional Conference Organisers (PCOs) needed to be collected and disclosed by member companies, as per Edition 8.4 of the IPHA Code. (PCOs are described in the Code as ‘a company / individual specialised in the organisation and management of congresses, conferences, seminars and similar events’). The only organisations that could be identified by Pharma Integrity from the various company disclosures were:
· Critical Care Training Ltd. (BMS)
· Global Teamwork Ltd (BMS & Grünenthal)
· Hannon Oncology Education (Amgen)
· Health Ireland / Health One User Group (LEO Pharma, GSK, Lundbeck, Daiichi Sankyo)
· Healthcare Minds Ltd. (AstraZeneca)
· NB Medical Education (Lundbeck)
It is highly unlikely that these are the only PCOs used by IPHA member companies in 2019, so companies should take the opportunity to be reminded of this obligation and consider whether an update to their internal disclosure process is required to capture and publish this information.
Conclusion
The data published on transferofvalue.ie is done so in a manner which makes it difficult to draw any meaningful comparisons. This is a result of variances in data entry between different companies, and a lack of data matching of submitted data by IPHA. The transferofvalue.ie website was never designed to be a dynamic portal, and the limitation of this decision was recognised and acknowledged by involved parties at the time of its creation. Developing a dynamic portal would have cost a lot more in terms of cash and resource for the additional technical development, ongoing management, and oversight within IPHA. The website fulfils the requirements of the EFPIA Code, which had an eager drive for transparency within the industry across Europe at the beginning of the last decade. Moving forwards, even the introduction of discrete columns in the disclosure template to separate data by “Title”, “First name” and “Surname”, and a QC of data by companies to eliminate typos before submission would help with analysis of the data in Ireland.


